
|
|
Pipelines are One Kind of Heaven - for SRB at least I started to write this article for selfish reasons. I wanted to improve my understanding of SRB organisms, and in particular why they should be so damaging to pipelines. But the more I read, the more intrigued I became, until I eventually concluded that there might be value to the wider pipeline community in attempting to summarise what I had read, but interpreted towards the particularly damaging effects of grooving in water injection pipelines. I had previously seen SRB as insidious destroyers of value, but I now see remarkable opportunists with a strong life-force, and I marvel at the important role such humble organisms play in the cycle of life. In the course of researching this piece, I found a wide range of explanations for some of the phenomena that are seen, each with an emphasis based on the author's particular interests; and whilst most were generally supportive in their conclusions, a number were contradictory. I have therefore chosen to offer my own interpretation and I would welcome comments that help my understanding further. I hope to update this article from time to time as my knowledge develops. Remarkable Organisms Sulphate Reducing Bacteria are remarkable organisms. They exist far from the sun, at the bottom of the food chain - yet they play a vital part in those final few steps in which organic materials are oxidised back to the basic components from which they were assembled during photosynthesis by plants and algae. They recycle carbon as carbon dioxide, and sulphur as mineral deposits. They have evolved to live in some of the most inhospitable places imaginable to man; absent of oxygen, absent of light, buried in slime and ooze, and under extremes of pressure in the deep sea or pipelines; yet they thrive. 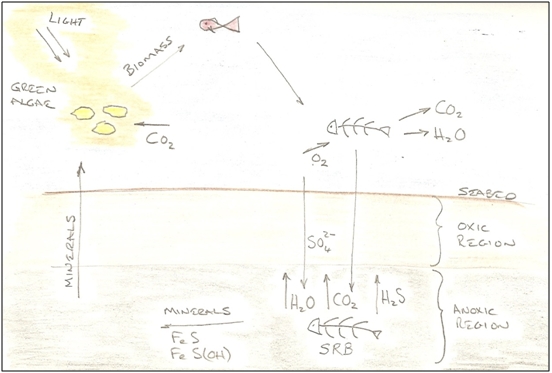
Figure 1 - The Role of SRB in the Cycle of Life Given the primitive nature of bacteria and their tiny size (typically 1µm to 5µm long), one might expect each species to evolve to fit a highly specific niche; using specific nutrients, and producing specific intermediate and waste products, with rich mixtures of such organisms living in colonies to digest organic materials completely. According to Wikipedia, as of 2009, 220 species of sulphate reducing bacteria were known[1], and it appears widely accepted that many of them live in complex syntrophic systems to optimise their mutual life-chances. As if the environmental circumstances in which they live are not challenging enough, one imagines that the nutrient flux must also be quite low, lying buried in a cold seabed waiting for some organic matter to be passed down the food chain. Their way of acquiring energy for life, is to reduce sulphate (SO42-) (which is plentiful in sea water), to sulphide (S2-), and to do this they need a supply of electrons. The sulphide ion is highly reactive and reacts with hydrogen to form hydrogen sulphide, so this is the SRB's main by-product. However the hydrogen sulphide reacts with iron to form a number of iron sulphide minerals, so a balance is struck whereby hydrogen sulphide and iron sulphides are both formed in prodigious quantities. Only a modest proportion of the iron that dissolves is precipitated as iron sulphide. Most is precipitated as iron carbonate using carbon dioxide produced by the SRB, and bicarbonate ions dissolved in the water. Hydrogen sulphide is highly toxic, but the SRB are protected from excessive toxicity by the beneficial effects of the sulphide reacting with the metal ions in the water, to precipitate out of solution. A number of SRB species also consume hydrogen, and their management of the hydrogen ion supply may influence the ratio of hydrogen sulphide to metal sulphide. It also seems likely that the colony will have evolved internal transport mechanisms to help extract this toxin and reject it into the passing water stream. Prising the oxygen away from the sulphate requires a supply of electrons, and the exact means by which the SRB achieve this remains unknown, but a consequence in pipelines is that the electrons come from the steel, causing it to dissolve. The end products are water, carbon dioxide and hydrogen sulphide (and some left-over organic matter, if not completely consumed). Whatever process the SRB use to reduce sulphate, it has a low energy yield; perhaps 5% of what a respiring (oxygen breathing) organism could have extracted from the same fuel source[2]. A healthy colony must therefore consume a tremendous amount of sulphate and organic matter relative to its mass, and a tremendous amount of energy remains bound-up in the H2S waste. Moving Home Every so often, the seabed in which the colony resides is disturbed, and the colony becomes dispersed. Each individual SRB must then fend for itself whilst it lives for some time in a planktonic state, seeking out a new micro-niche in which to settle. During the planktonic phase of the bacterium's life, it adopts 'survival mode', as it lives without the support of the colony, and its own preferred nutrients are now free-floating. It is also hopelessly out-competed by the much higher energy yielding oxygen respiring micro-organisms. It must feel like heaven then, for such a bacterium, to suddenly find itself inside a water injection pipeline. Instead of a low nutrient flux determined by seabed permeability and internal colony transport mechanisms, there is suddenly a super-abundance of sulphate and organic material literally rushing past. Not only that, but the source water has likely been enriched with organic nutrients and raised in temperature by the human activities and operational process at inlet. The SRB thus enter our pipeline, and as bottom dwellers with limited means of self-propulsion, settle where they can on the bottom, or in some sheltered spot where the shear stress from the passing water can't drag them to oblivion. 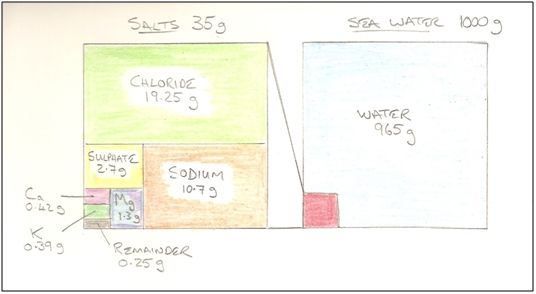
Figure 2 - Composition of Sea Water As can be seen from Figure 2, sulphate is plentiful in sea water, comprising about 0.3% of its mass. It will also be noted that iron does not appear in the figure. It is hardly present in sea water, at only 0.0034ppm, so most of the iron within the scales is from the pipeline itself. A pipeline transporting 25 mbd (thousand Barrels per day) of sea water will transport 10 tonnes of sulphate per day, and as the atomic mass of the sulphate ion is 96 g/mol, these 10 tonnes equate to approximately 100,000 mol of sulphate. Taking a representative sulphate reduction rate of 10 fmol cell-1 day-1 [3], one can calculate that the above sulphate supply could theoretically support a population of 1.0E+19 SRB, and if one assumes a maximum population density of one SRB per unit volume one body length in each direction, and a body length of 5µm, the supportable volume of SRB would be 1,250m3. A typical 10-inch water injection pipeline has an internal surface area of 0.75m2/m, so if the maximum population density film described was 0.2mm thick everywhere, the pipeline would need to be more than 8,000km long before all sulphate was consumed. It therefore seems likely that if there is a nutrient limitation, it is in the supply or organic matter rather than sulphate. But that has not been estimated here. Establishing a Biofilm/Reef inside the Pipeline Whilst the term 'biofilm' is frequently used to describe the SRB colony, this shouldn't be taken to imply a passive or sedentary place. It is literally teeming with life, with many of the species present capable of reproducing themselves every few hours under ideal conditions. It's more like a jungle, where there is both intense competition for resources, but also syntrophic relationships that provide mutual support. A more apt description for a marine context might be 'reef'. But in this case, the substrata in which they live is not the skeletal remains of more complex organisms, but the steel's surface itself (and the mineral scales that form as a by-product of their metabolism). The steel initially provides a difficult environment because it is relatively smooth and coated with oxides, so the electro-chemical process that later become so damaging are difficult to establish. The SRB presumably also need some time to revert from their free-floating 'survival mode' to one where they are established as a colony. Many species also secrete extracellular polymeric substances (EPS) i.e. 'slime', to help them bind and function, and this also takes time to establish. Eventually, the surface of the steel becomes roughened and the colony's grip becomes more tenacious. Even later, as the layers of corrosion product build up, one can imagine a relatively porous structure of various iron minerals encrusting the pipe wall, with its pore spaces occupied by the SRB biomass, yielding a secure environment for a massive population, with a massive surface area to best exploit the nutrient laden water flowing by. The colony's composition might also be expected to change depending on location within the 'reef'. The dominant species in the outer layers in direct contact with the passing water would differ to those in direct contact with the steel. For example, the dominant SRB in the outer layers might be most adept at utilising organic nutrients in the water such as volatile fatty acids and alcohols, whilst those on the steel's surface would consume hydrogen from the corrosion process they drive. Indeed, the outer layers of the colony may host other types of (non SRB) fermentative bacteria that break down the available organic polymer materials into forms more digestible to the underlying SRB. 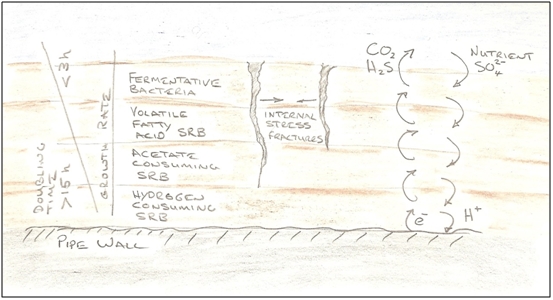
Figure 3 - A 'Reef' of SRB and Corrosion Product Note that if Figure 3 represented a cross section of film only 0.1mm thick, then an individual SRB would be about the size of the charge sign on the electron at bottom right. Corrosion at Micro-Scale The corrosion processes caused by SRB are not well understood, but an important mechanism is cathodic depolarisation. Cathodic depolarisation proceeds with the SRB acting as electron pumps. They provide the conditions whereby electrons can be won from the surface of the pipe (at a cathode). These electrons can then be provided to the sulphate, so that it can be reduced to sulphide, and the SRB do this by consuming hydrogen. A deficiency of hydrogen at the metal surface presumably encourages hydrogen ions to dissociate from water. These ions are positively charged, and will attract electrons out of the steel to form atomic hydrogen. For every two electrons taken, one atom of steel dissolves as an iron ion (at the anode). The oxygen stripped from the sulphate as it is reduced is used in part to produce carbon dioxide, and hydrogen ions combine with the sulphide to form H2S. The CO2 and H2S dissolve in the water and lower its pH, making more hydrogen ions available. Other species in the water also bind with the mineral scale to provide a complex mix of minerals. An electro-chemical cell is thus established, with the SRB responsible for maintaining the health of the cathode by consuming the H2 that is produced there, and an electrical current then flows in the pipe wall between anode and cathode locations. This mechanism provides an additional route for the oxidation of the steel (and production of hydrogen) in corrosive environments. The process of hydrogen ions accepting electrons is called reduction - the opposite of oxidation, which is the loss of electrons by the steel. Every electrochemical reaction must have these two half reactions. A reaction will be spontaneous if the sum of the electrode potentials of the half reactions is positive. By definition the standard hydrogen electrode cell has a value of 0 V (it is the reference potential used to calculate all other cell potentials). The oxidation potential for Fe → Fe2+ is +0.44 V, making the overall reaction potential positive which is why a shiny bare steel surface can corrode in an acidic environment without the presence of SRBs, producing hydrogen gas. The metal surface acts locally as both cathode and anode. 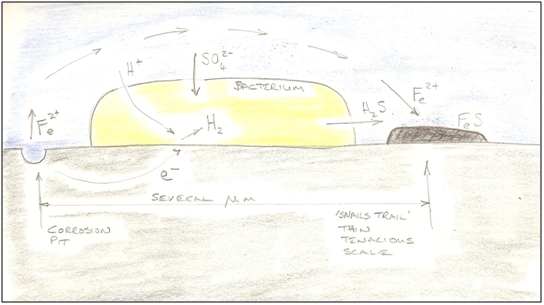
Figure 4 - Postulated Corrosion Mechanism at Cellular Scale The sulphide ions are highly reactive. They combine with iron or hydrogen ions to form iron sulphide and hydrogen sulphide, or more complex minerals incorporating hydroxide and carbonate ions that are also present. The simplest iron sulphides are themselves highly corrosive, but as they age and transform to more complex forms, their corrosivity diminishes. As the hydrogen sulphide converts into iron iron sulphide, two H+ ions are released, making more H+ available to pick electrons off the metal surface. As the iron sulphide formation process takes place at a bacterial scale, the films that form are thin and relatively uniform. They are intimate and tenacious. The first electro-chemical cells were of bacterial dimension (of the order of a few micron between anode and cathode); but as the iron sulphide mineral scales are not good electrical conductors, the resistivity of the circuit very quickly increases and the reaction slows to a virtual stop once the scale bridges the gaps between the cell sites. The resistivity of steel is 1.5E-7 Ωm, whilst that of sea water is 2.0E-1 Ωm, so the electrons prefer to flow through the pipe. The resistivity of the iron sulphide scales is more difficult to estimate, but the SRB themselves within the mineral pore spaces are not good conductors. If the resistivity of 'massive sulphide ores' is representative, at between 1.0 Ωm to 1.0E-2 Ωm [4], then it can be seen that the scales are probably 4 orders of magnitude more resistive than the pipe. In a linear circuit, the resistivity of 0.1mm of scale would be equivalent to 1.0m of pipe wall, so electrons will travel significant distances in search of a flow path. The circuit geometry inside the pipe is clearly not linear, and no attempt to analyse the actual circuit has been attempted here, but it seems reasonable to assume that the top of the pipe is easily within their reach. Corrosion at Macro-Scale When a breach in the iron sulphide film occurs, localised corrosion is very aggressive. It is driven by the electro-chemical cell described previously, but now on a massively wider scale. The distance between anode and cathode is now of the order of centimetres, and can draw on the hydrogen-consuming appetites of literally millions of SRB. The driving potential moves from the infinitesimal to the damaging, as the ratio between the anode and cathode is large. 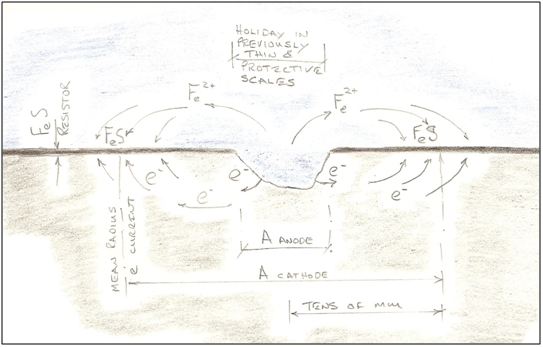
Figure 5 - Postulated Mechanism for Pitting Corrosion Iron and sulphur are remarkable elements and can form a wide range of minerals together, and as the scales age, their composition changes and may even come to contain growth ring features reflecting the life of the colony over time.
The changing composition and density of the scale will create internal stresses, so that once the scale becomes suitably thick, it can be expected to become friable, and it is at this stage that conditions are ripe for the formation of grooving, which inevitably means that the pipeline's days are numbered. Grooving Corrosion Pipelines that have suffered grooving corrosion invariably have very thick scales over their upper portion. Scale thicknesses of 0.1mm close to the groove, and 0.5mm to 3.5mm away from the groove have been reported[5]. The internal stresses within the very thick scales causes them to break up and fall as platelets, and given their high iron content, they are very dense and sink quickly. Although the platelets are dense, they are easily transported by the flowing water by sliding and tumbling along the bottom of the pipe, scouring it clean. The scouring action creates a bright shiny metal surface which enhances the efficiency of the anode, leading to the production of massive amounts of hydrogen at the SRB colony/cathode, and this allows the corrosion processes to hit top gear, with corrosion rates of 1.0 to 2.5mm per year being typical. The centre of the cathode is at top dead centre, which seems counter-intuitive at first, as the colony would normally prefer to live on the bottom and that is where the scales might be expected to form, but two competing explanations are offered for this:
The thick scales provide a large volume within which the SRB can live securely, so there are a number of reinforcing effects. The thick scale layers become stressed, so more platelets are encouraged to form, huge amounts of hydrogen are being made available. The SRB population thrives, and there is a net migration of iron upwards. Some proportion of the steel from the groove is re-deposited as an iron mineral on the roof of the pipeline, and the roof of the pipe barely corrodes further, merely bearing the scars of isolated pits created before the groove became established. The roof of the pipe becomes very rough, so the flow there is turbulent, and the turbulence helps dislodge more scale. 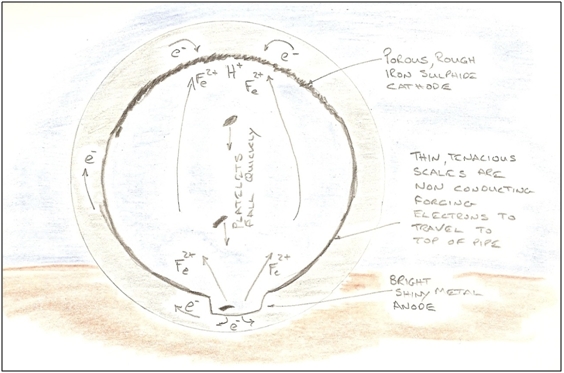
Figure 6 - Postulated Mechanism for Grooving Corrosion One thus finds that scales in grooved pipes become thicker towards the top of the pipe, nearly always to the point that ultrasound is unable to penetrate them, and inspection efforts become re-focused at identifying their inner surface. However, as has been described, these surfaces are so rough and spongy, that this too is a very challenging exercise. Extensive cleaning is required prior to inspection. The groove formation process is thus strongly influenced by gravity. Where the pipeline flows up hill, one might expect the platelets to slow and become less energetic, and when the pipeline runs downhill, the platelets may tend to lift off. Whether these topography changes are sufficient to initiate or terminate the groove would depend on the general (horizontal) flow conditions in that vicinity. Similarly, the groove becomes more difficult to maintain at bends, because the paths of the individual platelets becomes less well defined. An arresting feature of the grooves is their extreme definition. They have sharply defined edges and flatter bottoms than the curvature of the pipe. In many ways they have similar forms to river banks carved through soft soils, so it may be that the groove formation is also driven by erosion-corrosion mechanisms sparked during contacts between the platelets and the bottom of the groove. As there is a strong electrochemical relationship between the anode and cathode, one might expect there to be relationships between the size and shape of the groove (anode) and the size and shape of the cathodic scales. It would be interesting to see a finite element analysis of the electrical fields and currents flowing within a pipe cross section! Implications for Integrity Management Water injection pipelines generally fail because of grooving corrosion. Corrosion rates in the groove can be very aggressive, so those responsible for integrity management must be very diligent, or the pipeline will fail prematurely. Designers of new pipeline systems should recognise the challenge of grooving corrosion and either provide systems for preventing the groove from forming, such as effective water treatment, pigging, and inspection facilities (and operators need to use them diligently), or they should avoid the problem altogether by materials selection, such as by specifying plastic lined pipe. In practice it would appear very difficult to prevent grooving, as the majority of water injection pipelines are grooved (although there are a few examples of long-lived pipelines without grooves). Operators responsible for managing unlined pipelines have a difficult problem. There are no monitoring techniques that can inform whether grooving is active or not (although some means of catching platelets or listening for them could be attempted), so inspection is required; and there always seem to be reasons to defer these activities, and 'unexpected' failures occur with disappointing regularity. The thick scales provide a very protective environment from biocides, so these treatments need to be carefully designed, and ideally applied in conjunction with some mechanical agitation. If a groove has formed, the volume of scale could be considerable. Consideration should be given to potential downstream effects before attempting to the clean the pipeline. For example, the scales may reduce injectivity close to the wellbore. The author is hopeful too that one day, a method of refurbishing such pipelines in situ will become available. Environmental Impacts of Cleaning It will be recalled that a variety of sulphurous minerals are formed by SRB activity, and that some of these are highly reactive. When they are discharged to the sea, they quickly grab all of the available oxygen dissolved in the water, so the effects can be very damaging to marine life in the area. Certain minerals will change from black to red in colour and may give a visually dramatic plume. Conclusions The conditions for groove formation are quite specific:
 References
Bibliography
| ||||||||||||||||||||||||||||||||||||||||||||||